The largest clinical trial programme ever conducted in adult patients with PKU1
More than 350 patients participated in the extensive clinical trial programme that established the efficacy, safety and dosing regimen of PALYNZIQ®.
Phase 3 programme: PRISM-1 and PRISM-2 studies1–3
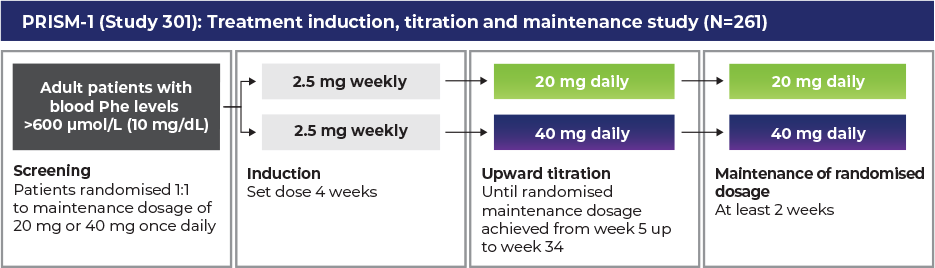
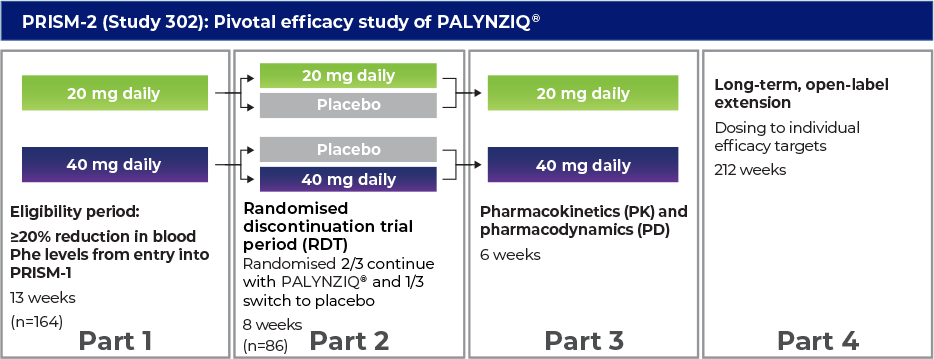
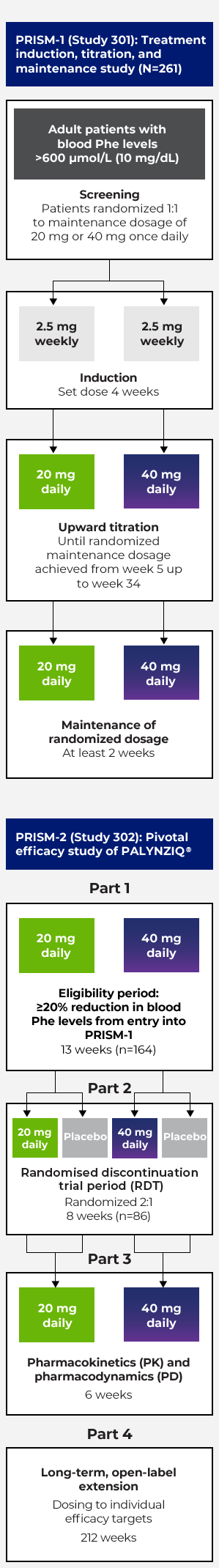
Randomised discontinuation trial period (RDT)
- The pivotal efficacy study was designed as an RDT, an 8-week, double-blind, placebo-controlled trial in which patients were randomised 2:1 to either continue their randomised dosage of PALYNZIQ® (
20 mg or40 mg once daily) or receive matching placebo4 - Entry into the RDT was determined by whether patients achieved a ≥20% reduction in blood Phe levels from pretreatment baseline, following a period of up to 13 weeks of additional treatment with PALYNZIQ®4
- Of the 164 patients who entered
PRISM-2 , 86 (52%) met this target and continued to the RDT4
- Of the 164 patients who entered
Primary endpoint: Change in blood Phe levels from randomised withdrawal baseline to week 8 between patients randomised to PALYNZIQ® and patients randomised to placebo4
Secondary endpoints: Change in inattention and mood symptoms, as measured by the ADHD
Patient baseline characteristics1
- The majority of patients were not on a Phe-restricted diet prior to and during the study*
Patient demographics and characteristics at treatment-naive baseline (ITT population; N=261)1
ITT, intent-to-treat.
*A Phe-restricted diet is defined as >75% of protein intake from medical food.4
†The European Food Safety Authority (EFSA) recommendation for total protein intake from natural food is 0.83 g/kg for adults, which equals 58 g/day for a 70-kg person.5
References: 1. Thomas J et al. Mol Genet Metab 2018;124(1):27–38. 2. Thomas J et al. Mol Genet Metab 2018;124(1):27–38 [supplementary material]. 3. PALYNZIQ® Summary of Product Characteristics. 4. Harding CO et al. Mol Genet Metab 2018;124(1):20–26. 5. European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for protein. EFSA J 2012;10(2):2557. doi:10.2903/j.efsa.2012.2557.